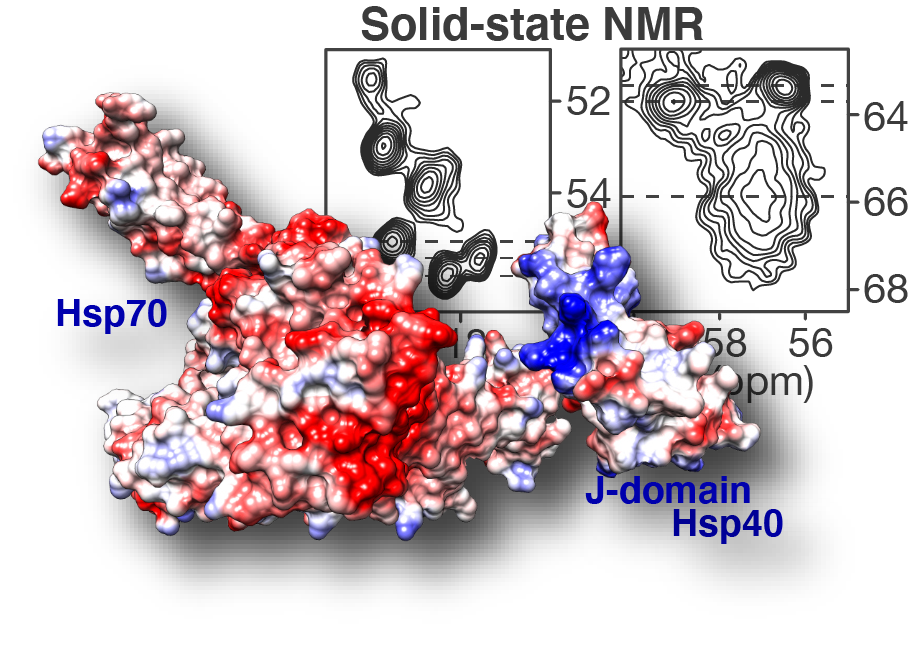
Congratulations to our postdoc Dr. Irina Matlahov and our collaborators in the group of Prof. Lukasz Joachimiak at UTSW! Our new paper on the structure and function of an oligomer chaperone prohibiting polyglutamine aggregation has now been published in the journal Nature Communications. In it, we deploy a combination of solid-state NMR (ssNMR), solution NMR, cross-linking mass spectrometry (XL-MS) and other methods to visualise and understand these Hsp40 DnaJB8. This so-called co-chaperone teams up with Hsp70 to prevent polyglutamine aggregation in cells, but has been hard to understand. This is because it has itself a strong propensity to self-aggregate (or phase separate), even in a cellular context. Here we deploy a range of methods to identify and characterise an interesting and important feature of this multi domain protein. We show that the protein blocks its own activity when it is just “sitting around”, but can be activated due to environmental triggers. We figure out the specific amino acids are involved, and find that similar interactions are apparently present in other analogous chaperones. The intriguing possibility now arises that this self-blocking allows the chaperones to sit around in a “dormant” state until their action is (urgently?) needed. Then, perhaps, substrate proteins that could cause disease could be bound and that this binding interaction would quickly activate a bunch of these proteins. Further experiments will be needed to test this idea, and whether it can be used to control or activate these innate protective mechanisms as part of disease treatments in the future. Note that polyglutamine proteins aggregate in many incurable diseases, including Huntington’s disease (HD).
For more information, please read our new paper at the journal:
Ryder, B.D.; Matlahov, I.; Bali, S.; Vaquer-Alicea, J.; Van der Wel, P.C.A. & Joachimiak, L.A. (2021) Regulatory inter-domain interactions influence Hsp70 recruitment to the DnaJB8 chaperone. Nat. Commun. 12: 946 [DOI: https://doi.org/10.1038/s41467-021-21147-x]
Our work for this paper was made supported by the American NIH funding our polyglutamine research and instrumentation (grants GM112678 & OD012213-01). Nowadays, our polyglutamine research continues in Groningen with funding from CampagneTeam Huntington and CHDI. Note that this paper is published “open access” like all of our (recent) HD related work.
Note added:
The paper also received some online attention in some science-oriented news sites, based on press releases by the universities involved. This includes a highlight on the HD News website on April 20th.