Author: Patrick

Funding: project grant from CampagneTeam Huntington
We are very grateful for the continued support from the Dutch CampagneTeam Huntington foundation (CTH), which has just announced that they will fund our new project grant on Huntington’s disease (HD). This is a project together with our longstanding collaborator Prof Amalia Dolga (at the GRIP institute of the University of Groningen). It is an extension of an earlier project that the CTH funded, and which paid for the PhD research project of Dr. Greeshma Jain. She published several papers from her research on the structures and toxic properties of HD-related protein aggregates and how they are modulated by aggregation […]

Postdoc position available – ssNMR/DNP
We are looking for a postdoctoral researcher to join the group, funded by our new NWO WINC grant. See the application site of the university for now. The Solid-state NMR group of Prof Patrick van der Wel at the University of Groningen is looking for a postdoctoral researcher with experience in magic-angle-spinning (MAS) solid-state NMR spectroscopy and/or dynamic nuclear polarization (DNP). The multiyear postdoctoral position is funded by the Dutch national funding organization NWO, via a national consortium grant that establishes a new ssNMR/DNP facility in Groningen. The postdoc will join the Solid-state NMR group supervised by Prof. Patrick van […]
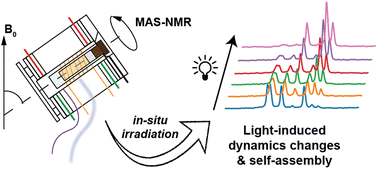
Publication: In-situ irradiated MAS NMR to probe dynamic transitions.
We are excited to report the publication of our paper on in-situ illumination during MAS NMR in the Journal of Materials Chemistry A. This publication is the culmination of a long project that found its origins quite a few years ago, even before the lab moved from Pittsburgh to Groningen. Photochemistry and optogenetics are increasingly influential topics in both chemical and biological research, respectively. The application of illumination and light triggers can be used to control chemical and biological processes. In this paper we focus on the intersection of such processes and materials science – demonstrating how magic-angle-spinning (MAS) NMR […]

New Publication: Silk-inspired hybrid materials – ssNMR and more.
Congratulations to PhD student Raffaella Parlato, as well as our collaborators from the group of Marleen Kamperman, with the publication of a new collaborative paper in the journal Communications Chemistry. Raffaella used solid-state NMR to study the structure and dynamics of (labeled) polypeptides within the hybrid polymer-peptide materials prepared by the Kamperman group. The employed peptides are poly-alanine peptides inspired by the structural motifs found in proteins in spider silk. Spider silk proteins use polyalanine segments to form stable higher order structures that enable the remarkable materials properties of silk. Here, these peptides were used to change the materials properties […]
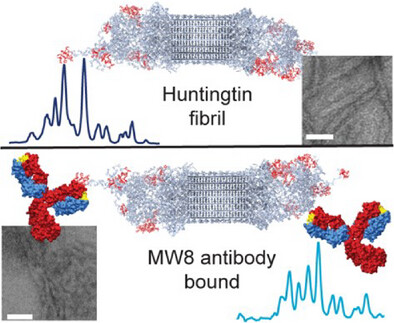
Publication: how antibodies bind to the fuzzy coat of Huntingtin protein fibrils.
Congratulations to Raffaella for the publication of her second first-author paper, this time on the use of NMR spectroscopy to study how antibodies bind to the aggregated state of huntingtin protein fragments, relevant to Huntington’s disease. This work is part of our ongoing research on the biochemistry of Huntington’s disease, and was supported by funds from the European Huntington Disease Network EHDN and the CampagneTeam Huntington. Antibodies are widely used as diagnostic tools in HD research, and also are considered as potential treatment modalities. An example of the latter approach can be found in the case of Alzheimer’s disease, where […]

Publication: Impact of aggregation inhibitors on protein aggregation and aggregate toxicity.
Congratulations to Greeshma Jain, Marina Trombetta-Lima and the whole team of co-authors for the publication of our most recent paper on Huntington’s disease (HD), in the journal Nature Communications. This paper is actually the fourteenth peer-reviewed publication from the lab on HD research, since our first paper in 2011. It was enabled by crucial funding from the CampagneTeam Huntington foundation, who supported our HD research program when the Van der Wel group moved back to the Netherlands. In this new report we describe our use of structural analysis (NMR and EM) to look how aggregation-inhibiting small molecules (polyphenols) change the […]

Webinar: GlycoScience Webinar on polysaccharide ssNMR research
On 27 March Patrick gave an online presentation in the EGC webinar series, on the topic of using solid-state NMR (and isotope labeling) to study polysaccharides in various contexts. The title of the presentation was: “Labeling and solid-state NMR studies of hyaluronic acid and other polysaccharides: hydrogels, nanoparticles and more” EGC Webinar #86 Glycochemistry (March 27, 4 pm CET) Patrick van der Wel (Zernike Institute for Advanced Materials, University of Groningen, the Netherlands) “Labeling and solid-state NMR studies of hyaluronic acid and other polysaccharides: hydrogels, nanoparticles and more” A recording of the lecture is visible via YouTube. In the presentation […]
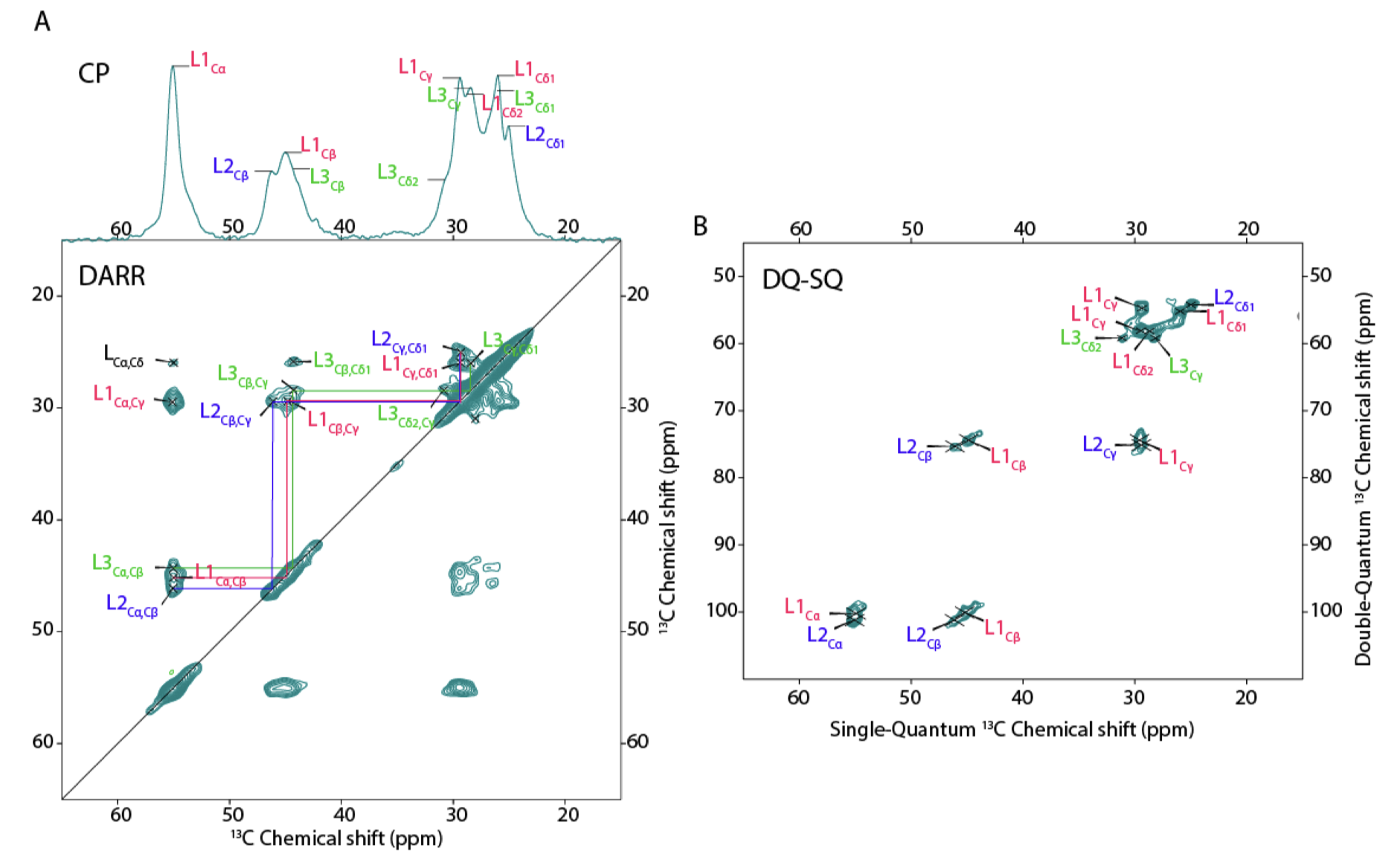
Publication: Peptide-based self-assemblers and self-replicators (with insights from ssNMR).
Congratulations to our collaborators in the group of Sijbren Otto (RUG), and Alessia, for the new paper in the journal Chem. This report discusses how self-assembling and self-replicating molecules can borrow principles from polypeptide amyloid formation. In particular, the concept of ‘steric zippers’ proves to be highly relevant – an idea from the amyloid field that comes out of seminal work by the group of David Eisenberg (UCLA). Our contributions in this new paper relate to the use of 1D and 2D solid-state NMR analysis of the self-assembled materials, in samples where targeted isotope-labeled amino acids were incorporated. With these […]
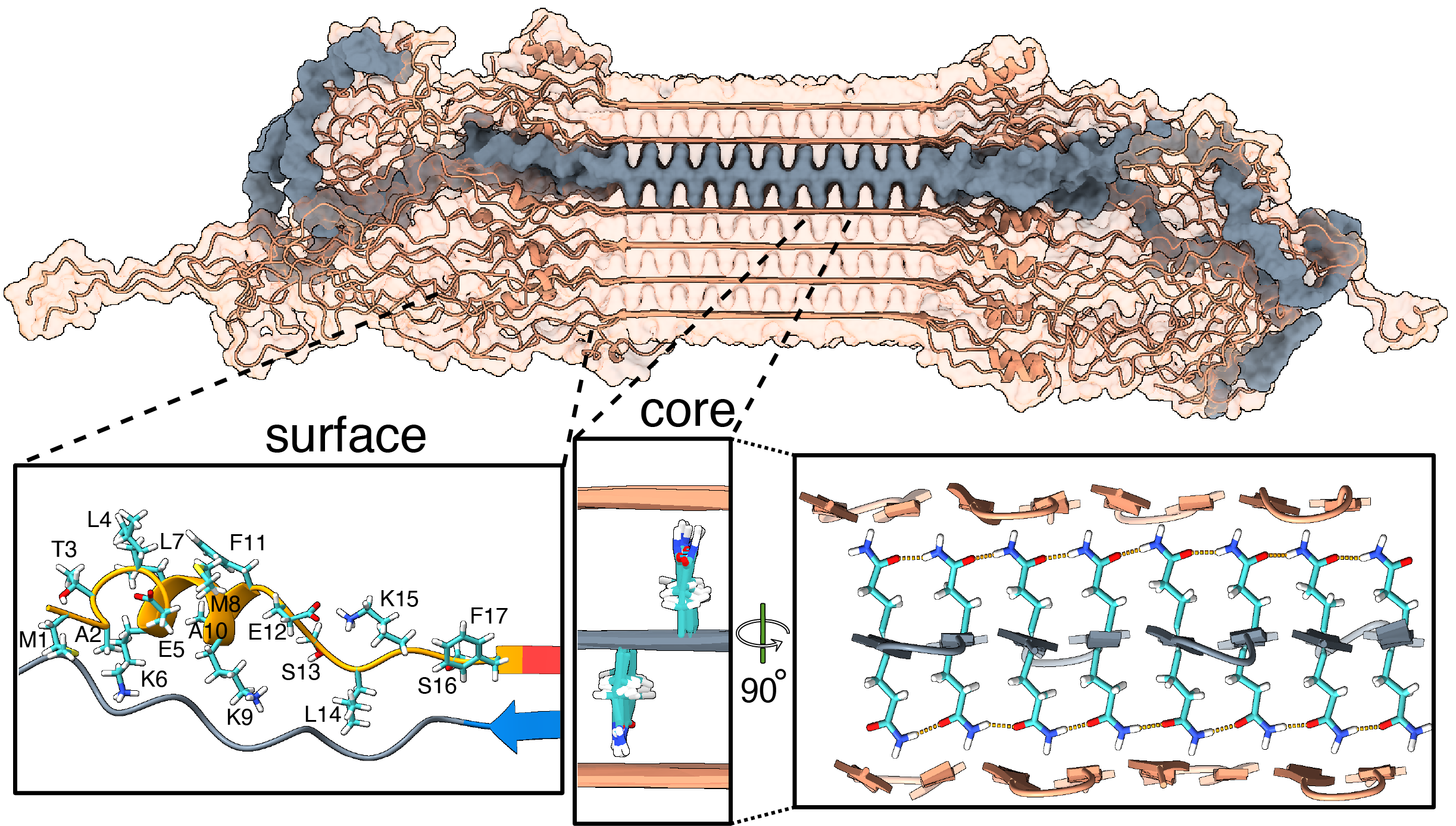
Publication: Structure of protein aggregates implicated in Huntington disease
Congratulations to Irina, Greeshma, and Raffaella, together with our international network of collaborators, on a newly published paper that came out in Nature Communications in Dec 2024, in a special collection on the Biology of rare genetic disorders. In this collaborative work, we looked at the structure of proteins behind Huntington’s disease (HD), enabled by crucial funding from the CampagneTeam Huntington and the CHDI Foundation. HD is an inherited neurodegenerative disease, with patients having a mutated form of the huntingtin (HTT) gene. The mutation affects a repeating sequence of CAG codons that has been expanded in HD patients. This translates […]