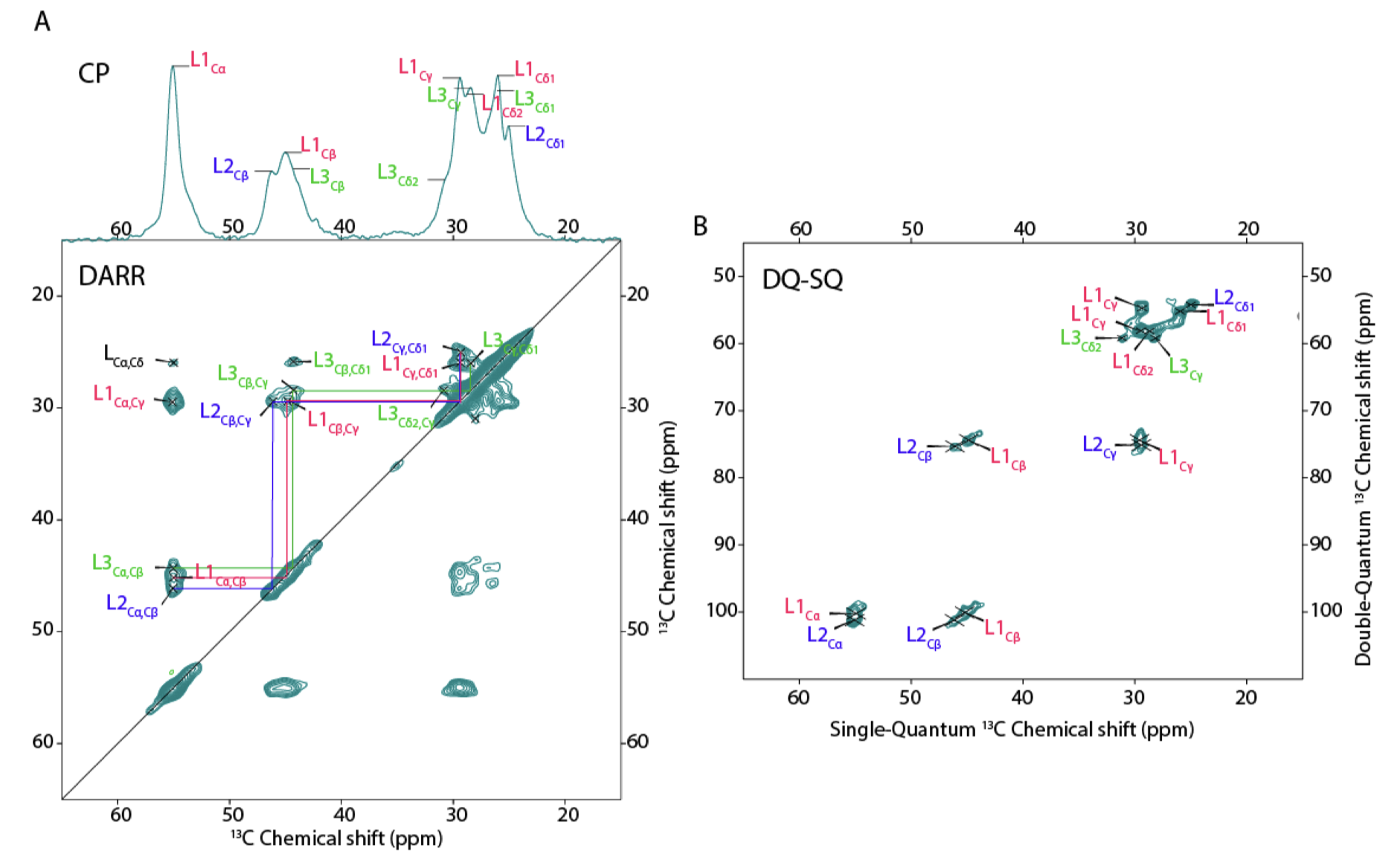
Congratulations to our collaborators in the group of Sijbren Otto (RUG), and Alessia, for the new paper in the journal Chem. This report discusses how self-assembling and self-replicating molecules can borrow principles from polypeptide amyloid formation. In particular, the concept of ‘steric zippers’ proves to be highly relevant – an idea from the amyloid field that comes out of seminal work by the group of David Eisenberg (UCLA). Our contributions in this new paper relate to the use of 1D and 2D solid-state NMR analysis of the self-assembled materials, in samples where targeted isotope-labeled amino acids were incorporated. With these NMR studies we were able to probe the structure and symmetry of the assembled state, giving some insights into the fibril architecture. See also the figure below, from the paper’s SI.
Citation: Eleveld, M.J., Wu, J., Liu, K., Ottelé, J., Markovitch, O., Kiani, A., et al. (2025) Departure from randomness: Evolution of self-replicators that can self-sort through steric zipper formation. Chem 102374 https://doi.org/10.1016/j.chempr.2024.11.012
PS. Conceptually and practically, this work has similarities to our earlier JACS paper with the group of Nat Rosi at the University of Pittsburgh, in which we helped detail the steric zipper motifs in a different class of peptide-chimera fibril-forming molecules. Also that paper may be interesting to revisit! [DOI https://doi.org/10.1021/jacs.6b07322]