Blog Posts
News: Dutch-language radio
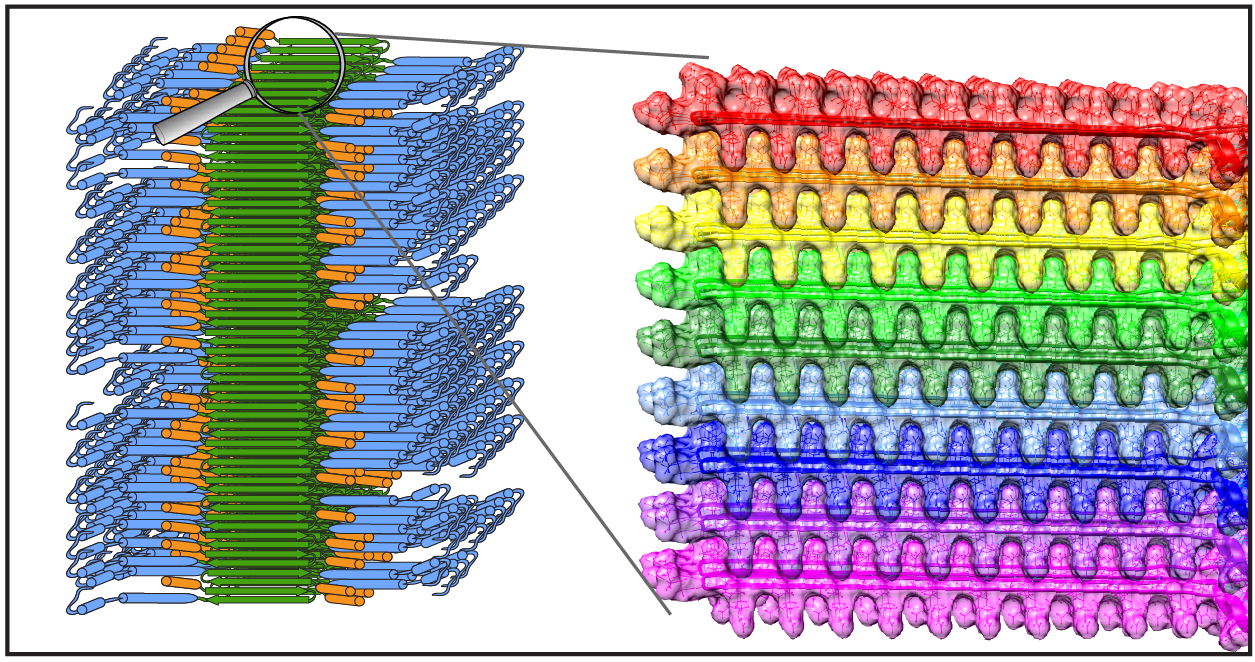
Recently our research was covered (briefly) on a Dutch radio station, featuring an interview with Patrick. It is mostly about our work applying ssNMR to studying how proteins aggregate in Huntington’s disease. You can find the recording online at the NPO radio website. More information about our Huntington disease research can also be found on this page, and in two recent webinars.

Upcoming event: BPS networking event on polyQ biophysics
Hereby an early announcement of an upcoming online event hosted by our group and the group of Markus Miettinen: “Biophysics of polyglutamine aggregation: how does it start and how does it end?“
This is an online event is organized in context of the networking event series sponsored and organized by the Biophysical Society (BPS). Our event aims to bring together international scientists working on the biophysics of polyglutamine (polyQ) protein aggregation. The event will take place on July 6th 2022, from 15:00-18:00 CET.
More information can be found at the page from the BPS or our own institute. An online registration page is planned to online at the BPS, see below. If you want more information in the mean time, please contact us by email.
Update May 2022: the registration page is now active at the BPS. The link is here. Note that this does require a free BPS account to work. Let us know if you have any issues or questions.
Publication: SSNMR of alginate hydrogel (re)hydration
A new open-access publication by Mustapha and collaborators has been published in the journal Food Hydrocolloids, describing how he used various ssNMR measurements to probe alginate hydrogel structure and (re)hydration. Alginates can be cross-linked with calcium to form hydrogels, which are used in many different types of applications. This includes their use in slow-release drug delivery as well as food/nutritional applications. In this new paper, Mustapha shows how 1H, 2H and 13C ssNMR measurements can be used to see how these hydrogels are structured, but especially also how they are hydrated by the aqueous solvent. A key feature of the work is the use of 1H MAS NMR to detect the water molecules in the hydrogel macropores as well as those waters directly interacting with the alginate. The two types of water molecules form distinct ‘pools’ in the hydrogel, which can be nicely distinguished by their different molecular motion. The latter is detected via different relaxation measurements, in these NMR experiments.
To read more:
El Hariri El Nokab, M..; Lasorsa, A.; Sebakhy, K. O.; Picchioni, F.; van der Wel, P. C. A. Solid-State NMR Spectroscopy Insights for Resolving Different Water Pools in Alginate Hydrogels. Food Hydrocolloids 2022, 107500.
Webinar: Huntington’s disease mechanisms.
On Sept 28 2021, Patrick gave a public webinar on the topic of protein Misfolding and aggregation in Huntington’s disease (and related polyglutamine disorders). This was part of the international webinar series on Cellular and Protein Homeostasis. This webinar is now available for streaming online at the following URL. The webinar discusses some of the lessons we learned from our ongoing studies of what happens to mutated proteins in Huntington’s disease. In particular: what is the role of the mutated polyglutamine domain? How does it change its conformation as it transitions from its normal fold to a disease-causing “misfolded” state?
Title: On the role of beta-hairpins in the nucleation and propagation of polyglutamine protein aggregation.
For more information see also our recent papers in JMB (2020) and EBM (2019).
Publication: Structural and motional changes in a cytochrome c – lipid complex implicated in apoptosis.
Congratulations to lab alum Dr. Mingyue Li and our collaborators on the publication of a new paper in the Journal of Molecular Biology. The paper is online via its DOI link. The paper describes how we used solid-state NMR spectroscopy to characterise the partial destabilization of the native fold of the protein cytochrome c, as it is bound to cardiolipin lipids. This protein-lipid complex is implicated in the process of programmed cell death, where it plays a key role in triggering the self-destruction of undesired or disease cells in higher organisms. Notably, the CL-bound protein catalyses the process of mitochondrial lipid peroxidation, which we see being reconstituted in vitro via mass-spectrometry lipidomics. The latter work is done by our longstanding collaborators in the group of Valerian Kagan at the University of Pittsburgh. The current paper builds on our earlier work, but is of especial interest based on the fact that we bridge some of our prior findings (that suggested the protein to be surprisingly “folded” on the membrane) to studies that report a greater degree of mobility of the CL-bound protein. Here we see how the experimental conditions regulate protein mobility and also pinpoint how different extents of mobility are present in the membrane-bound cytochrome c. Interestingly, the regional dynamics map partly, but not completely onto the previously identified “foldons” that define the folding landscape of this widely studied mitochondrial protein. For more details see the paper below. It is available as #openaccess so just follow the DOI link for access:
Reference:
Mingyue Li, Wanyang Sun, Vladimir A. Tyurin, Maria DeLucia, Jinwoo Ahn, Valerian E. Kagan, Patrick C.A. van der Wel (2021) Activation of Cytochrome C Peroxidase Function Through Coordinated Foldon Loop Dynamics upon Interaction with Anionic Lipids, Journal of Molecular Biology, Volume 433, Issue 15, 23 July 2021, 167057
Publication: New paper on mitochondrial protein-lipid interactions published in PNAS.
Congratulations to lab alumns Dr. Abshishek Mandal and Dr. Jennifer Boatz, as well as our collaborators in the USA and Spain! A new collaborative paper on mitochondrial protein-lipid interactions has just been published in the journal PNAS. In this multidisciplinary work we studied how the protein Drp1 binds the special mitochondrial lipid cardiolipin in order to do its job managing the proper fission of mitochondrial membrane. Our collaborator Rajesh Ramachandran (at Case Western) coordinated a wide array of experimental and computational approaches to determine how Drp1’s “variable domain” (VD) binds cardiolipin. Along the way, two apparent CL-binding motifs were detected, which are seemingly shared by other CL-binding proteins. Their mutation disrupts CL binding and also the proper management of mitochondrial morphology, pointing to the importance of CL-based signals and interactions in this vital cellular process.
Experimentally, our group contributed various solid-state NMR measurements of the lipids, with which we probe the specificity of the interactions and also observe how the protein modulates the lipid bilayer itself. Moreover, we were involved in the sequence/structure analysis: sequence analysis of the “disordered” VD domain pinpointed those parts of the structure most likely to engage in lipid-driven folding upon membrane binding. The “MoRF” motifs (“molecular recognition features”) indeed appear to be involved in CL binding, based on NMR and mutational studies. In Drp1 they are intimately involved in a folding transition of the VD domain upon membrane binding. It will be interesting to see how widespread these CL-binding motifs (CBMs) are in other CL-binding proteins.
Reference:
[1] Mahajan M, Bharambe N, Shang Y, Lu B, Mandal A, Madan Mohan P, et al. NMR identification of a conserved Drp1 cardiolipin-binding motif essential for stress-induced mitochondrial fission. Proc Natl Acad Sci USA2021;118:e2023079118. https://doi.org/10.1073/pnas.2023079118.
Webinar: Structural biology of Huntington’s disease.
On May 15th 2021, Patrick gave an invited lecture in the online webinar series on the Molecular Bases of Proteinopathies hosted by the Ramamoorthy lab at the University of Michigan. Patrick talked about our research on polyglutamine protein aggregation and the structural biology of Huntington’s disease.The seminar title was “The Structural Biology of Protein Misfolding in Huntington’s disease”. If you are interested, the seminar has been posted to YouTube, where it can be viewed here.
Preprint: cytochrome c – cardiolipin studies by ssNMR on bioRxiv
Our latest update on structural studies of this peroxidase-active protein-lipid complex implicated in mitochondrial apoptosis has now posted to bioRxiv: https://www.biorxiv.org/content/10.1101/2021.02.24.432556v1
In the preprint we discuss how ssNMR reveals the involvement of specific and localised dynamics in the lipid-bound protein. Interestingly, the mobility is dependent on the bound lipid species, with an apparent correlation to the resulting peroxidase activity. The lipids thus act as both substrates and regulators of the pro-apoptotic enzymatic activity of the protein. This was also discussed in the recent webinar as discussed in an earlier post.
This work was made possible by several great collaborators at the University of Pittsburgh, and funding from the NIH for both the project and employed instrumentation.
Update: The paper has now been accepted for publication in the Journal of Molecular Biology, and can be found online at its DOI link.
Reference:
Mingyue Li, Wanyang Sun, Vladimir A. Tyurin, Maria DeLucia, Jinwoo Ahn, Valerian E. Kagan, Patrick C.A. van der Wel (2021) Activation of Cytochrome C Peroxidase Function Through Coordinated Foldon Loop Dynamics upon Interaction with Anionic Lipids, Journal of Molecular Biology, in press
Webinar: Talk on dynamics ssNMR studies in the Emerging Topics in Biomolecular Magnetic Resonance series
On Thu Feb 18th, Patrick was invited to give a talk in the online Emerging Topics in Biomolecular Magnetic Resonance webinar series. This is a global online seminar series featuring the frontier of research using magnetic resonance spectroscopy (mostly NMR) in biological systems. The webinars are also posted (most of them at least) to the dedicated YouTube channel. Lots of great cutting edge research there!
Patrick presented a combination of published an unpublished research that focused on the studies of how lipid (per)oxidation is catalysed in mitochondria, as part of the mitochondrial apoptotic process. Whilst oxidation of biomolecules (lipids, DNA, proteins) is commonly seen as simply an undesirable negative site effect, recent studies show that in apoptotic mitochondria the process of lipid oxidation is actually being catalysed (and directed!) by a protein-driven enzymatic reaction. We have been studying the underlying molecular events for some years now, resulting in a number of related papers [1-4]. In these papers we initially emphasised how the protein involved (cytochrome c) is surprisingly similar in structure to its native state [1]. In more recent work we are figuring out how this native state is variably “mobilised” in its peroxidase active state, and how this is regulated by the substrate of the peroxidation reaction [3]. This latter story is the focus of the online talk.
The idea of the lipids acting as both substrate and dynamic activator, was introduced in our recent paper in Structure [3]. A paper is forthcoming that will look at this in more detail, with some data shown and discussed in the talk. The talk is now also available on the YouTube channel of the webinar series. (If you go there, hang around for many more interesting talks, including the talk by Roland Riek on automating NMR assignments with new computational approaches, in the same video.)
This research has been supported by funds from the NIH to my group and the group of collaborator Valerian Kagan. The papers below should be available (mostly) as open access, but please requests reprints from us if needed.
Some related papers from the lab:
- Mandal, A.; Hoop, C. L.; DeLucia, M.; Kodali, R.; Kagan, V. E.; Ahn, J.; van der Wel, P. C. A. Structural Changes and Proapoptotic Peroxidase Activity of Cardiolipin-Bound Mitochondrial Cytochrome c. Biophys. J.2015, 109(9), 1873–1884. https://doi.org/10.1016/j.bpj.2015.09.016.
- Our first studies of the CytC-CL complex by ssNMR.
- Mandal, A.; Van der Wel, P. C. A. MAS (1)H NMR Probes Freezing Point Depression of Water and Liquid-Gel Phase Transitions in Liposomes. Biophys. J.2016, 111 (9), 1965–1973. https://doi.org/10.1016/j.bpj.2016.09.027.
- A question was asked about phase transitions. We look at this by ssNMR in this paper. Also earlier work on VDAC may be relevant.
- Li, M.; Mandal, A.; Tyurin, V. A.; DeLucia, M.; Ahn, J.; Kagan, V. E.; van der Wel, P. C. A. Surface-Binding to Cardiolipin Nanodomains Triggers Cytochrome c Pro-Apoptotic Peroxidase Activity via Localized Dynamics. Structure2019, 27 (5), 806-815.e4. https://doi.org/10.1016/j.str.2019.02.007.
- Kagan, V. E.; Tyurina, Y. Y.; Sun, W. Y.; Vlasova, L. L.; Dar, H.; Tyurin, V. A.; Amoscato, A. A.; Mallampalli, R.; van der Wel, P. C. A.; He, R. R.; Shvedova, A. A.; Gabrilovich, D.; Bayir, H. Redox Phospholipidomics of Enzymatically Generated Oxygenated Phospholipids as Specific Signals of Programmed Cell Death. Free Radic Biol Med2020, 147, 231–241. https://doi.org/10.1016/j.freeradbiomed.2019.12.028.
- Talks about the role of purposely catalysed enzymatic lipid peroxidation as a signal in biology.
Publication: paper on an anti-polyglutamine oligomeric chaperone studies published!
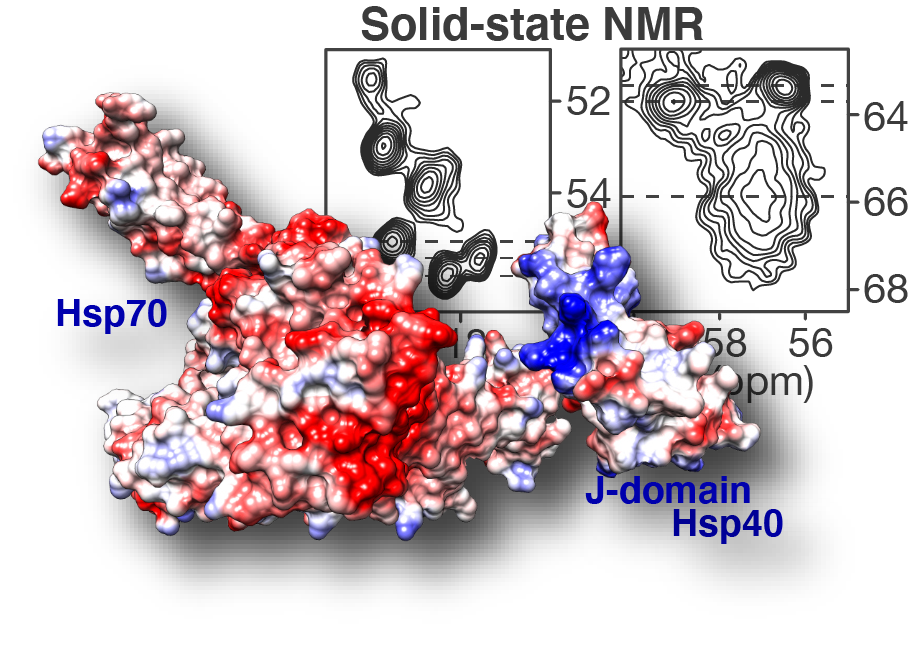
Congratulations to our postdoc Dr. Irina Matlahov and our collaborators in the group of Prof. Lukasz Joachimiak at UTSW! Our new paper on the structure and function of an oligomer chaperone prohibiting polyglutamine aggregation has now been published in the journal Nature Communications. In it, we deploy a combination of solid-state NMR (ssNMR), solution NMR, cross-linking mass spectrometry (XL-MS) and other methods to visualise and understand these Hsp40 DnaJB8. This so-called co-chaperone teams up with Hsp70 to prevent polyglutamine aggregation in cells, but has been hard to understand. This is because it has itself a strong propensity to self-aggregate (or phase separate), even in a cellular context. Here we deploy a range of methods to identify and characterise an interesting and important feature of this multi domain protein. We show that the protein blocks its own activity when it is just “sitting around”, but can be activated due to environmental triggers. We figure out the specific amino acids are involved, and find that similar interactions are apparently present in other analogous chaperones. The intriguing possibility now arises that this self-blocking allows the chaperones to sit around in a “dormant” state until their action is (urgently?) needed. Then, perhaps, substrate proteins that could cause disease could be bound and that this binding interaction would quickly activate a bunch of these proteins. Further experiments will be needed to test this idea, and whether it can be used to control or activate these innate protective mechanisms as part of disease treatments in the future. Note that polyglutamine proteins aggregate in many incurable diseases, including Huntington’s disease (HD).

For more information, please read our new paper at the journal:
Ryder, B.D.; Matlahov, I.; Bali, S.; Vaquer-Alicea, J.; Van der Wel, P.C.A. & Joachimiak, L.A. (2021) Regulatory inter-domain interactions influence Hsp70 recruitment to the DnaJB8 chaperone. Nat. Commun. 12: 946 [DOI: https://doi.org/10.1038/s41467-021-21147-x]
Our work for this paper was made supported by the American NIH funding our polyglutamine research and instrumentation (grants GM112678 & OD012213-01). Nowadays, our polyglutamine research continues in Groningen with funding from CampagneTeam Huntington and CHDI. Note that this paper is published “open access” like all of our (recent) HD related work.
Note added:
The paper also received some online attention in some science-oriented news sites, based on press releases by the universities involved. This includes a highlight on the HD News website on April 20th.